Is Epilepsy a Progressive Neurodegenerative Disease? Evidence From a Large Multicentre Longitudinal Neuroimaging Study
Abstract number :
2.184
Submission category :
5. Neuro Imaging / 5A. Structural Imaging
Year :
2018
Submission ID :
502499
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Marian Galovic, University College London; Victor Van Dooren, University College London; Tjardo Postma, University Medical Center Utrecht; Sjoerd B. Vos, Centre for Medical Image Computing, University College London; Epilepsy Society, Chalfont St Peter; G
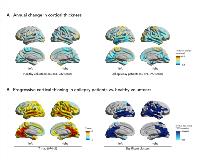
Rationale: It is controversial whether epilepsy is a static or progressive disease. Knowledge on disease progression has potentially important implications for rapid diagnosis and early treatment. The majority of previous neuroimaging studies in epilepsy were cross-sectional and their design did not permit to directly detect progressive morphological changes. The small number of longitudinal studies was restricted to small cohorts and they did not directly compare epilepsy patients with healthy controls, failing to differentiate epilepsy disease progression from normal aging. We performed a large longitudinal neuroimaging study of progressive brain atrophy in epilepsy. Methods: We studied chronic focal epilepsy patients without cerebral mass lesions and compared them with age- and sex-matched healthy volunteers from three independent cohorts. All subjects received two or more high-resolution structural MRI scans on the same scanner using identical sequences at least 6 months apart (mean interval 2.5 ± 1.6 years). We compared whole-brain cortical thickness estimates using linear mixed-effects models adjusted for age, sex, and interscan intervals and corrected for multiple comparisons. Results: We included 191 chronic focal epilepsy patients with 399 MRI scans and compared them to 141 matched healthy volunteers with 282 scans. Epilepsy patients showed a widespread highly significant pattern of progressive atrophy, mainly involving the bilateral temporal lobes, medial parietal and occipital cortices, paracentral gyri, and opercula (Fig. 1B). The yearly cortical thinning rate in epilepsy patients was twice the amount of age-related thinning observed in healthy volunteers (0.022 ± 0.067 mm/year vs. 0.011 ± 0.029 mm/year, p<10-14, Fig. 1A). Distinct patterns of progressive atrophy were demonstrated for temporal (n=102) vs. frontal (28) lobe epilepsy and for left (n=73) vs. right (n=68) lateralized cases. Significant progressive atrophy in these subgroups was most pronounced ipsilaterally to the epileptic focus but typically also affected a widespread area extending beyond the epileptic focus and frequently impacted the contralateral hemisphere. Progressive atrophy was not associated with seizure frequency or antiepileptic drug (AED) load and longitudinal cortical thinning did not differ between seizure-free and non-seizure-free patients. Significantly accelerated longitudinal atrophy was detected in those with early onset of epilepsy, longer disease duration, and hippocampal sclerosis. There were no regions of more progressive atrophy in healthy controls when compared to epilepsy patients. Conclusions: In the largest longitudinal neuroimaging study in epilepsy, we demonstrated that morphological abnormalities in epilepsy are dynamic rather than static, with yearly atrophy rates twice that of normal aging. The patterns of progressive neurodegeneration in focal epilepsy were syndrome specific and widespread, typically spreading to regions beyond the epileptic focus. Progressive cortical thinning was associated with disease duration but not with seizures or AED intake, which suggests that disease progression is a seizure-independent phenomenon. Our results are in support of rapid treatment and early epilepsy surgery to prevent ongoing neurodegeneration. Funding: Medical Research Council (MR/L013215/1)