Listening to the Community Voice in a Traumatic Brain Injury Study to Prevent Post-Traumatic Epilepsy: Preliminary Findings of the EpiBioS4Rx Public Engagement Core
Abstract number :
1.417
Submission category :
13. Health Services (Delivery of Care, Access to Care, Health Care Models)
Year :
2018
Submission ID :
505904
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Daniel Correa, Albert Einstein College of Medicine; Churl-Su Kwon, Icahn School of Medicine at Mount Sinai; Solomon L. Moshé, Albert Einstein College of Medicine; and Nathalie Jette, Icahn School of Medicine at Mount Sinai
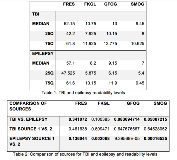
Rationale: Recruitment and retention of TBI participants in the ICU pose challenges due to: 1) surrogate consenting in an emotionally stressful environment, 2) need to educate surrogate decision-maker about abstract concepts such as epilepsy prevention and 3) challenges of retention due to care transition. The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) study is a National Institute of Neurologic Disorders and Stroke Center Without Walls designed to accelerate development of prevention therapies for post-traumatic epilepsy (PTE). The study includes a Public Engagement Core (PEC). We describe our initial steps in engaging patients, surrogate-decision makers, scientific and consumer organizations, using participatory action research (PAR) to facilitate the development of effective recruitment and retention strategies for a future PTE prevention randomized controlled trial (RCT). Methods: A mixed-methods approach was used including development of: 1) webinar on PAR methods, 2) public outreach and education materials and 3) TBI community focus groups. As part of the development of educational materials, a review of publicly available education content (>100 files from 12 organizations) about TBI and epilepsy was completed. The readability of 10 TBI and 10 epilepsy websites or online handouts was assessed(Flesch Reading Ease Score (FRES), Flesch-Kincaid Grade Level (FKGL), Gunning Frequency of Gobbledygook (GFOG), and Simple Measure of Gobbledygook (SMOG)). Mean scores were evaluated against national recommendations, between each source, between TBI and epilepsy related resources using analysis of variance, t tests or nonparametric equivalents, as appropriate. Results: A 30-minute webinar was developed to provide background for the PEC members about PAR. TBI materials had the following readability levels (results recorded as median (25th%ile;75th%ile): FRES-52.2 (42.2;61.8), FKGL-10.8 (7.9;11.9), GFOG-13 (10.2;13.8), SMOG-9.5 (8.0;10.6). Epilepsy materials had: FRES-57.1 (47.5;61.6), FKGL-8.2 (5.9;10.2), GFOG-9.2 (6.2;11.9), SMOG-7 (5.4;9.5). Comparisons of the materials in the sources of epilepsy were significantly different in FKGL, GFOG, SMOG (all p<0.01). No statistical difference was seen comparing TBI material sources. Our initial brain injury focus groups raised concerns about the timing of research team approaching families due to stressors when they arrive in the ICU and concerns about study retention due to care transition from ICU to another site/facility. Consortium members, raised the need (amongst other concerns) for cultural/linguistic relevance when developing educational and recruitment materials. Conclusions: This initiative represents an ongoing collaboration with multilevel stakeholders from both the TBI and epilepsy communities to inform the design of a future RCT aimed at preventing PTE. Educational materials related to TBI and epilepsy will be essential. Preliminary analysis of existing materials demonstrates substantial variability in their comprehension level. Based on the PEC’s ongoing experience, it is recommended PAR be considered for future RCTs. Funding: This work is supported by NIH grants 1U54NS100064 EpiBioS4Rx, Center Without Walls and Einstein-Montefiore CTSA Grant Number UL1TR001073.