Long-Term Safety and Efficacy of Add-on Cannabidiol (CBD) Treatment in Patients with Lennox-Gastaut Syndrome (LGS) in an Open-Label Extension (OLE) Trial (GWPCARE5)
Abstract number :
1.298
Submission category :
7. Antiepileptic Drugs / 7B. Clinical Trials
Year :
2018
Submission ID :
500065
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Anup D. Patel, Nationwide Children's Hospital; Antonio Gil-Nagel, Hospital Ruber Internacional; Richard Chin, Royal Hospital for Sick Children; Wendy Mitchell, Children's Hospital Los Angeles; M. Scott Perry, Cook Children's Medical Center; Arie Weinstock
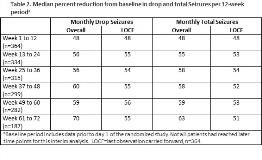
Rationale: LGS is a rare epileptic encephalopathy that is often treatment-resistant. Efficacy and safety of CBD in the treatment of seizures associated with LGS was demonstrated in two phase 3 controlled trials, GWPCARE3 (NCT02224560) and GWPCARE4 (NCT02224690). Here we report a second interim analysis of the OLE of these trials, designed to assess long-term safety and efficacy of CBD added to existing antiepileptic drug (AED) treatment in children and adults with LGS. Methods: Patients who completed either 14-wk double-blind, randomized controlled trial could enter this OLE study (NCT02224573), in which they received a plant-derived pharmaceutical formulation of highly purified CBD (100 mg/mL) in oral solution for up to 3 yrs. Initially, patients were titrated to 20 mg/kg/d administered in two divided doses, which could then be decreased or increased to 30 mg/kg/d at the investigator’s discretion. Primary endpoint was safety with secondary endpoints of drop seizure and total seizure frequency, and Subject/Caregiver Global Impression of Change (S/CGIC). Results: Of 368 patients with LGS who completed the core studies, 366 (99%) enrolled in the OLE and median follow-up was 61 wks (range 3 d to 87 wks); some patients had not reached the later treatment windows, and 88 (24%) had withdrawn at the time of this analysis. Mean age was 16 yrs, with 33% =18 yrs, and 54% male. Patients were taking a median of 3 concomitant AEDs (52% clobazam; 38% valproic acid [VPA]). At prerandomization baseline, patients had median 80 drop seizures and median 168 total seizures per 28 d. Mean modal dose of CBD during the OLE treatment phase was 23 mg/kg/d (range 21–25 mg/kg/d across the 12-wk visit windows). During the extended follow-up, adverse events (AEs) occurred in 94% of patients, serious AEs were reported in 33%, and 11% discontinued due to AEs (see Table 1 for most common). Forty-seven patients (13%) had elevations in liver transaminases >3× upper limit of normal, with none meeting Hy’s law criteria for severe liver injury; 35 (74%) of these patients were taking concomitant VPA. There were 5 deaths (1%), none deemed related to CBD treatment by the investigator(s). Median % reductions in drop and total seizures, assessed at 12-wk visit windows, were maintained through 72 wks, ranging from 48%–70% for drop seizures and 48%–63% for total seizures (Table 2); last observation carried forward analyses showed similar findings. Approximately 88% of patients/caregivers reported an improvement in overall condition on the S/CGIC at weeks 24 and 48. Conclusions: Long-term treatment with add-on CBD was generally well tolerated, with a similar AE profile to that observed in the core studies. CBD resulted in maintained reductions in drop and total seizure frequency through 72 wks of exposure, with a high proportion of patients/caregivers reporting an improvement in overall condition. Overall, these results demonstrate long-term benefit of CBD and durability of effect in patients with LGS. Funding: GW Research Ltd
.tmb-.jpg?Culture=en&sfvrsn=9e95d513_0)