Optimising Trajectories in Computer-Assisted Planning for Cranial Laser Interstitial Thermal Therapy: A Machine Learning Approach
Abstract number :
3.354
Submission category :
9. Surgery / 9C. All Ages
Year :
2018
Submission ID :
501202
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Kuo Li, University College London; Vejay N. Vakharia, University College London; Rachel Sparks, University College London; Lucas G. S. França, University College London; Alejandro Granados, University College London; Andrew W. McEvoy, University Coll
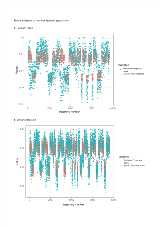
Rationale: Cranial laser interstitial thermal therapy (cLITT) is a first-line alternative to open surgery for drug resistant focal mesial temporal lobe epilepsy (MTLE). Recent studies have shown that ablation of the mesial hippocampal head and longitudinal cannulation of the hippocampus improve seizure freedom rates whilst better neuropsychological outcomes are associated with sparing of the parahippocampal gyrus (PHG). Optimal trajectory planning aims to avoid crossing vasculature, sulci and CSF cavities as well as maximising distance from vasculature. EpiNav:LiTT is a computer-assisted planning (CAP) platform that has been shown to significantly improve these metrics, but the optimal combination of entry and target zones has yet to be determined to maximize ablation of the amygdalohippocampal complex (AHC) whilst sparing the PHG. We apply a machine learning approach to predict ablation volumes from a variety of entry and target parameters and utilize these for CAP. Methods: Ten patients with hippocampal sclerosis (5 right) were identified from a prospectively managed database. CAP cLITT trajectories were generated using entry regions that include the inferior occipital, middle occipital, inferior temporal and middle temporal gyri. Target points were varied by sequential erosions and transformations of the centroid of the amygdala. In total 760 trajectory combinations were generated per patient and ablation volumes of the AHC and PHG were calculated based on a conservative 15 mm maximum ablation diameter. A composite ablation score of ablated AHC minus ablated PHG volumes were calculated and normalised per patient. Two machine learning approaches including random forest and linear regression were implemented to predict composite ablation scores and determine the optimal entry and target point combinations to maximize this. For the random forest 5 patients (3800 trajectories) were randomly allocated to the training dataset and the remaining 5 to the test set for validation. The regression analysis was based on all patients in the dataset. Results: The performance of linear regression was superior to random forest predictions with an R2 of 0.98 and 0.52 respectively (see Figure 1). Linear regression indicated that maximal composite ablation scores were associated with entry points that clustered around the junction of the inferior occipital, middle occipital and middle temporal gyri. The optimal target point was a translation of the centroid of the amygdala anteriorly and medially. The regression parameters were then used with CAP to further optimize distance from vasculature, intracerebral trajectory length and drilling angle to the skull (see Figure 2). Conclusions: Machine learning techniques accurately predict composite ablation scores with linear regression outperforming the random forest approach. Optimal CAP entry points for cLITT maximize ablation of the AHC and spare the PHG. These were found to cluster around the temporo-occipital junction and target the antero-mesial amygdala. Funding: Not applicable
.tmb-.png?Culture=en&sfvrsn=25288b40_0)