Predicting Seizure Risk from Interictal Epileptiform Activity
Abstract number :
3.084
Submission category :
2. Translational Research / 2B. Devices, Technologies, Stem Cells
Year :
2018
Submission ID :
502369
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Timothee Proix, Brown University; Wilson Trucculo, Brown University; Vikram R. Rao, University of California - San Francisco; and Maxime R. Baud, University Hospital Bern
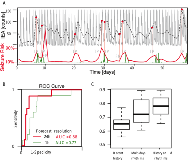
Rationale: The seemingly random occurrence of seizures is a source of suffering for patients with epilepsy as it leads to constant uncertainty. A reliable method to anticipate periods at risk of seizures would help these patients to control their seizures and improve their quality of life. Recent chronic EEG studies have shown that circadian and multidien (multi-day) rhythms of interictal epileptiform activity (IEA) strongly correlate with the likelihood of seizure occurrence. In this study, we assessed whether IEA rhythms can help predict epileptic seizures. Methods: Thirteen patients implanted with the NeuroPace RNS® System were selected based on availability of data. The RNS System includes eight electrodes that record hourly counts of IEAs and seizures. The recordings were performed over the span of several months (range 3-104, median 27.6). After interpolation of gaps in data, phase and amplitude of the circadian and multidien rhythms were extracted from the IEA time series. Data for each patient were divided sequentially in train, validation and test sets. Prediction was performed with a point process Generalized Linear Models (PP-GLMs) using conditional Poisson distribution. History (time-lagged values) of the seizure point and IEA count processes, as well as covariates of the circadian and multidien rhythms were introduced as features in the PP-GLM. Models were first optimized on the validation dataset, by selecting the length of history (number of time lags) in the seizure and IEA time series that maximized the area under the receiver-operating characteristic (ROC) curve. Models were then tested on the held-out test dataset. Metrics to evaluate our model performance included area under the ROC curve and the precision-recall curve (PRC). Results: Prediction scores were evaluated on an hour and day basis. An example of prediction time series is shown in Fig. 1A. The seizure likelihood strongly correlates with the multidien rhythms. Area under the ROC (Fig. 1B) and PRC curves were evaluated in both cases for each patient. In both cases, the phase of the multidien rhythms significantly improved the prediction scores compared to when only the recent history of seizures and IEA was used (average AUC: 0.66 versus 0.72 for recent history only versus using multidien rhythms, p<0.01, t-test, Fig. 1C). Best performance (average AUC: 0.76, SD: 0.09) was achieved by combining both recent history, circadian and multidien rhythms covariates. To address the imbalanced nature of the seizure data (rare events), we used PRC analysis, which was consistent with the AUC-based results (average PRC: 0.30 versus 0.47 for recent history only versus using multidien rhythms, t-test, p<0.01). Conclusions: We showed that circadian and multidien rhythms of IEA significantly improve the prediction scores of seizure likelihood as compared to seizure history alone. This confirms that IEA cycles at multiple scales are key biomarkers of fluctuating activity in epilepsy. These results support the inclusion of circadian and multidien rhythms in seizure prediction algorithms, with potential for improving quality of life for patients with epilepsy. Funding: We acknowledge support from the National Institute of Neurological Disorders and Stroke (NINDS), grant R01NS079533 (WT); the U.S. Department of Veterans Affairs, Merit Review Award I01RX000668 (WT); the Pablo J. Salame '88 Goldman Sachs endowed Assistant Professorship of Computational Neuroscience at Brown University (WT).