Real-Time Detection of Glutamate Dynamics in Normal and Impaired Brain Cells With Novel Microelectrode
Abstract number :
3.280
Submission category :
7. Antiepileptic Drugs / 7A. Animal Studies
Year :
2018
Submission ID :
508065
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Teresa A. Murray, Louisiana Tech University; Chao Tan, Louisiana Tech University; Jessica L. Scoggin, Louisiana Tech University; Nam Nguyen, Louisiana Tech University; Urna Kansakar, Louisiana Tech University; Shabnam Siddiqui, Louisiana Tech University;
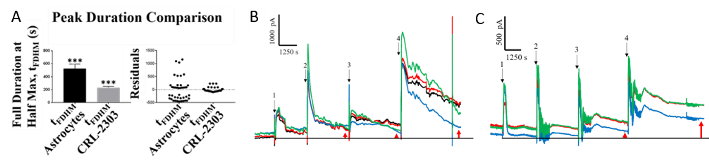
Rationale: Glutamate (Glu) signaling is dysregulated in diseases such as epilepsy and gliomas. Our goal is to develop probes enzyme-based platinum (Pt) microelectrode arrays (MEA) on ceramic probes have superior spatiotemporal resolution over microdialysis and single electrochemical probes. Methods: Highly sensitive Glu Pt-MEA probes (Glu microprobe) were created by applying L- Glu oxidase, bovine serum albumin, and glutaraldehyde to Pt-MEAs (R1, CenMeT) and then depositing m-phenylenediamine by cycling between ±0.25 V and ±0.75 V, 50 mV/s, 20 min. Microprobes had higher sensitivity, 4.6 ± 0.3 pA/µM (Fig. 1A), compared to the literature, due to increased loading of enzymes onto MEA sites. The microsensor had long-term stability, up to 1 mo, with minimal change in Glu sensitivity when stored in DI water at 22°C. Probe performance was assessed by discerning differences in uptake by astrocytes and uptake by CRL 2303 glioma cells. Measurements, including full duration at half maximum of concentration additions (FDHM, shown in Fig. 1A), rise (90-10%) and fall (20-80%) times, and fall velocity, were assessed using Origin Pro 2018. ANOVA and Welch’s correction were performed, and residual plots prepared using Prism v. 7.03. Residuals were used to show the effects of impaired Glu uptake by glioma cells. Results: We used the full duration at half maximum of the peak response (Fig. 1B) to additions of Glu into cell culture media and the fall time, or clearance time (Tc), of the Glu current, including analysis of residuals, to demonstrate that the probe had sufficient temporal resolution and glutamate sensitivity to discern differences between normal and impaired Glu uptake. A significant difference was found between the clearance rate (Tc) in astrocytes and glioma cells; p < 0.0001 (not shown). The Tc (mean ± SEM) for astrocytes was 1.3 ± 0.2 pA s 1 (n = 36 tests) while the Tc for the glioma cells was 3.3 ± 0.3 pA s 1 (n = 38 tests).Figure 1. (A) Glutamate response differed between healthy astrocytes and CRL-2303 glioma cells. Peak duration is shown, p < 0.001; rise and fall times were also significantly different. Notably, analysis of the residuals differed markedly. Residuals for astrocytes have 4 tightly-grouped levels that reflect the differences between the concentration levels. In contrast, the residuals for glioma cells do not have distinctly separate levels, suggesting that poor glutamate (Glu) uptake by these cells had relatively little effect on changing Glu concentration on the media. Four concentrations of Glu were used for establishing the calibration curve (not shown). B. Astrocytes take up increasing levels of Glu, whereas glioma cells (C) do not. Conclusions: We have developed a microprobe MEA that has high sensitivity for Glu and can be used for continuous monitoring of Glu dynamics in vitro in astrocytes and glioma cells. Given the long-term stability of the probes and their relatively high spatiotemporal resolution, these probes will be further developed for in vivo applications. Funding: This work was supported by a grant from the NSF (EPSCoR RII-2 FEC OIA1632891).