Rhythmic Patterns of Seizures and GABA in a Model of Temporal Lobe Epilepsy
Abstract number :
3.063
Submission category :
1. Basic Mechanisms / 1E. Models
Year :
2018
Submission ID :
502600
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Mani Ratnesh S. Sandhu, Yale University; Shaun E. Gruenbaum, Yale University; Roni Dhaher, Yale University; Hitten P. Zaveri, Yale University; Ketaki Deshpande, Yale University; and Tore Eid, Yale University
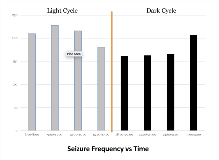
Rationale: Many physiological processes and diseases exhibit intriguing periodic (rhythmic) changes, such as cortisol secretion, body temperature, sleep/wakefulness, cancer, Alzheimer’s disease and epilepsy. It is well known that seizures can occur in clusters, followed by long periods of seizure freedom and many focal epilepsies exhibit circadian (~24 -hrs) vulnerability to seizure. The mechanism underlying circadian periodicity in seizure likelihood is not well understood. The objective of this study was to test a hypothesis linking periodicity in seizure likelihood to rhythmic changes in the metabolome of the brain. Methods: Seizures in a rodent model of mesial temporal lobe epilepsy (the methionine sulfoximine (MSO) model, n = 23), were detected through visual evaluation of the video-intracranial EEG record over 21 days. To collect extracellular fluid, a microdialysis probe was inserted in the dentate gyrus of the right hippocampus of this focal epilepsy model (n=7). Microdialysate was collected continuously in 1-hr fractions for up to 7 days. The sampled brain extracellular fluid was analyzed using liquid chromatography-tandem mass spectrometry(LC-MS/MS). The temporal distribution of seizures was assessed by evaluating seizure frequency in eight three-hour bins over 24 hours. The neurochemistry was similarly arrayed into eight three-hour bins. The distribution of seizures vs time and metabolome vs time was tested for non-uniformity using the chi-square statistic. Results: A total of 1535 seizures were documented by continuous video-icEEG. The seizure frequency of epileptic rats (n=23) was plotted over the 24-hr cycle, and a distinct rhythmic pattern was discovered (p < 0.0001, Fig. 1). There was a steady increase in seizure frequency during the light cycle (during sleep), and then a decrease towards the start of the dark cycle (the end of wake). With regard to neurochemistry, there was a striking rhythmic oscillation in GABA levels in the hippocampus of epileptic rats(p< 0.0001, Fig. 2).There was a steady decrease in GABA levels during the beginning of the light cycle and subsequent increase towards the end of the light cycle. The GABA levels continued to rise in the dark cycle, where seizure frequency was at its lowest and relatively stable. Conclusions: An increase in GABA levels over 24 hours corresponded with a concomitant decrease in seizure likelihood, while a decrease in GABA levels corresponded with an increase in seizure likelihood. This relationship suggests that the rhythmic oscillation of GABA may underlie the circadian vulnerability to seizure in these animals. Funding: Swebilius Foundation grant
.tmb-.png?Culture=en&sfvrsn=6bac8481_0)