Seizure-Induced Changes Within the Autonomic Nervous System
Abstract number :
2.047
Submission category :
3. Neurophysiology / 3C. Other Clinical EEG
Year :
2018
Submission ID :
502395
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Solveig Vieluf, Boston Children's Hospital, Harvard Medical School; and Institute of Sports Medicine, Paderborn University, Paderborn, Germany; Tobias Loddenkemper, Boston Children's Hospital, Harvard Medical School, Boston, MA, USA; Tanuj Hasija, Paderbo
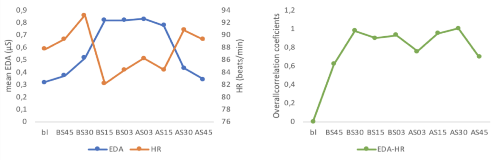
Rationale: Epilepsy-related dysfunction of the autonomic nervous system (ANS) is a prominent ictal, postictal, and interictal feature. Alterations of autonomic control in patients with epilepsy have specifically been demonstrated in cardiac and electrodermal modalities. Moreover, there has been some evidence that changes in ANS activity do not only occur within selected modalities, but follow a pattern across modalities that might reflect centrally driven integrative control processes. Therefore, we aimed to examine how electrodermal activity (EDA) and heart rate (HR) as well as their interaction change in relation to seizures. Methods: We included 13 patients (age: 15.2±4.96; 8 female) with generalized tonic-clonic seizures (onset: 10 focal, 3 generalized) during continuous video-EEG monitoring. Continuous EDA and heart rate (HR) were recorded by wireless multisensor devices (Empatica® E4, Milan, Italy). Nine 3-minute intervals were analyzed: baseline (20 minutes after start of measurement), 45-42, 30-27, 15-12 and 3-0 minutes before EEG seizure onset, as well as 0-3, 12-15, 27-30 and 42-45 min after EEG seizure offset. For unimodal analysis, mean values were calculated and analyzed by repeated measure ANOVA. For multimodal analysis, data was organized in matrices with the recordings of each participant as a column for each modality. Main features were extracted by principal component analysis and signal interactions were assessed by canonical correlation analysis (CCA). Significance of correlations was verified by hypothesis testing. An overall correlation coefficient was defined as Corr = 1 - i=1r(1-ki2), where ki2 denotes the squared canonical correlations. Results: Unimodal analysis revealed no significant main effect of time for EDA, F(8,96) = 1.43, p = .20, ?p2 = .11, or HR, F(8,96) = 0.35, p = .95, ?p2 = .03 (table 1). CCA analysis revealed that no significant interaction between modalities could be detected at baseline. Corr increased to high Corr values 30 min before seizure onset and remained at moderate to high levels after seizures. Conclusions: Differences in bimodal interactions between EDA and HR related to seizures occur at the patient group level and persist postictally, while unimodal analysis did not reach significance, possibly due to low patient numbers, acquisition of larger numbers is in progress. Bimodal interactions offer an interesting avenue for further research on seizure detection and prediction. Funding: The Epilepsy Research Fund
.tmb-.png?Culture=en&sfvrsn=314a32cc_0)