Swine Model for Validation of a Fully Integrated Closed-Loop Neuromodulation SoC With Wireless Power and Bidirectional Data Telemetry for Real-Time Seizure Control
Abstract number :
3.098
Submission category :
2. Translational Research / 2D. Models
Year :
2018
Submission ID :
502282
Source :
www.aesnet.org
Presentation date :
12/3/2018 1:55:12 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Yue-Loong Hsin, Chung Shan Medical University and Chung Shan Medical University Hospital; Cheng-Siu Chen, Chung Shan Medical University Hospital; Fu-Yuang Shih, Kaohsiung Chang Gung Memorial Hospital; and Syu-Jyun Peng, National Chiao Tung University
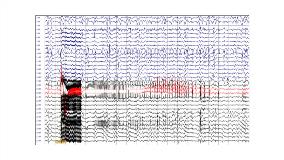
Rationale: A wireless telemetry designed system-on-chip (SoC) comprising of a 16-channel signal acquisition unit, a bio-signal processor and a 16-channel adaptive stimulator is under development for real-time seizure control. Accordingly, a big epilepsy animal model with higher brain volume is highly desired to enable detailed investigation of the seizure termination mechanism, and to validate the system in the stage of pre-clinical trial. Methods: In this research, domestic swine (8 pigs, 35-50 Kgs) were utilized. Pigs were induced with subsequent injection of azaperone, atropine, tiletamine and zolazepam; and were maintained with isoflurane. After craniectomy and dural opening, planar grid-electrodes implant was assessed.Subcortical injection (5 mm below brain surface) of penicillin (PCN) 5,000 IU induced periodic polyspike-wave discharges with time-locked myoclonic jerks. When the periodic epileptiform discharging was continuing, a transient of generalized fast activity with tonic convulsion could be reproduced by employment of 2 Hz electrocortical stimulation (ECS) (2 to 10 mA, 300µs pulse width, 2 seconds). Subsequently we applied PCN 5,000 IU every 30 minutes on the brain surface before ECS was conducted with different frequencies and without muscle relaxant injection to provoke ictal discharges in the subsequent pig studies.Then we tested the effective SoC-driving ECS paradigms (including current intensity, stimulation frequency, and timing) for seizure termination. Results: Thirty minutes after subcortical PCN injection, continuously rhythmic polyspike-wave discharges with time-locked jerks was evoked in the 1st and 2nd pigs. The waning magnitude of epileptiform discharges and jerking could be re-strengthened by subsequently repeated PCN injections. After we confirmed the reproducibility of ECS-induced seizures after PCN injected subcortically, we further found the advantages of brain surface application of PCN: rhythmic small spikes, and maintenance of susceptibility for ESC to induce seizures. Afterward, there would be constant duration electrical seizures (range from 16 to 18 seconds) responding to a 2-second train of 100 Hz ECS (3mA and 500 µs pulse width) in the 5th, 6th and 8th pigs. Each provoked seizure contained ictal onset, irregular and bursting phases subsequently.The SoC functionally terminated seizures that were provoked by 2 Hz ECS in the second and third pigs. Fortunately, the 100 Hz ECS provoked non-convulsive seizures with constant duration could be also early terminated by SoC-driving ECS. Each therapeutic stimulation parameter e.g., 15 Hz, 3mA and fixed 500 µs pulse width were repeatedly tested for 6 times. Between two different therapeutic parameter sessions, a non-therapy seizure would be induced to prevent the waning effect confounding the statistical comparison.Therapeutically, lower frequency (< 15 Hz) ECS shorten the seizure duration (mean 9.8 s vs non-therapy control 16.4 s, p<0.05) than higher frequency (50 Hz, 100 Hz and 150 Hz) ECS. The early seizure termination effect was only obtained by delivering therapeutic ECS impulse at the beginning of bursting phase. Conclusions: We successfully established an acute epilepsy model utilizing swine. Particularly, the seizures were not only reproducible but also maintained consistent features of electrical activity. By our preliminary results, therapeutic ECS may emphasize the seizure termination mechanism especially at early bursting phase. Funding: This work was financially supported by the Center for Neuromodulation Medical Electronics Systems from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
.tmb-.jpg?Culture=en&sfvrsn=a9b3b3fe_0)