Treatment Decision Support Systems (TDSS) in Epilepsy – Exploring Proxy Validity and Overall Clinical Outcome Success Related to TDSS-Predicted Antiepileptic Drug (AED) Choices
Abstract number :
1.323
Submission category :
7. Antiepileptic Drugs / 7E. Other
Year :
2018
Submission ID :
497176
Source :
www.aesnet.org
Presentation date :
12/1/2018 6:00:00 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Edward Han-Burgess, UCB Pharma; Ali Bozorg, UCB Pharma; John D. Hixson, University of California - San Francisco; Bosny Pierre-Louis, UCB Pharma; Shweta Joshi, CitiusTech; and Stephen Yates, UCB Pharma
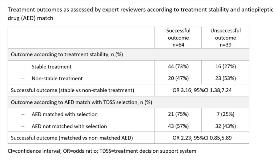
Rationale: Patient data are required for training computer-based algorithms of TDSS to make optimal predictions. To guide the development of a TDSS in epilepsy, a pilot study was conducted to explore whether data in electronic medical records (EMRs) and claims are of sufficient quality for evaluating treatment outcomes. Using evaluable records, we then sought to determine whether treatment stability, a proxy used for predicting treatment success approximated actual outcomes identified in records, and whether AEDs selected by the TDSS that matched actual AEDs in the records yielded successful outcomes. Methods: Anonymized EMRs combined with aggregated multichannel US claims data were obtained from HealthVerity; 149 combined records were randomly selected from 414 that met the inclusion criteria (patient age ≥18 years, epilepsy diagnosis, clinical history ≥1 year before and after AED start, >1-month treatment period and >1 physician note in that period). Records were stratified by stability and match. Stability was defined as continuous treatment with an AED for ≥1 year without addition of another AED (dose changes allowed). Match occurred when the AED was one of the top 3 selected by the TDSS. Five US-based epileptologists reviewed the records (29–30 each) to determine whether clinical outcomes (successful/unsuccessful) could be assessed. For the review, experts used their clinical judgment and were guided by a questionnaire developed specifically for the study comprised of commonly-considered elements of AED management (seizure frequency, tolerability, etc). Results: Of 149 records, reviewers were able to assess 103 (69%) as either successful or unsuccessful (Table); 46 (31%) were deemed ‘unable to assess’ due to reasons ranging from presence of complex comorbidities to insufficient data. Of 103 assessed records, 60 showed stable treatment, of which 44 (73%) had a successful and 16 (27%) an unsuccessful clinical outcome. Corresponding values for the 43 records with non-stable treatment were 20 (47%) and 23 (53%). Treatment stability was associated with a greater likelihood of success than non-stability (odds ratio 3.16; 95%CI 1.38,7.24). Of the 103 records, 28 had an AED that matched one of the TDSS-predicted AEDs; 21 (75%) had a successful and 7 (25%) an unsuccessful clinical outcome. Corresponding values for the 75 non-match records were 43 (57%) and 32 (43%). Match was associated with a greater likelihood of success than non-match (OR 2.23; 95%CI 0.85,5.89). Conclusions: Stable treatment with an AED for ≥1 year was associated with greater clinical success than non-stable treatment; therefore, treatment stability is a potentially suitable proxy for successful clinical outcome. AEDs matching the TDSS prediction also had a greater probability of success compared with non-matched AEDs. Interpretation is limited by the exploratory nature of the study and small sample size – a larger study powered for evaluation of treatment stability is warranted. Funding: UCB Pharma-sponsored