Characteristics of Adult Patients With Focal Seizure Receiving Eslicarbazepine Acetate Therapy in Routine Clinical Practice: Evidence From a Large US Commercial Claims Database
Abstract number :
2.263
Submission category :
7. Antiepileptic Drugs / 7C. Cohort Studies
Year :
2018
Submission ID :
500810
Source :
www.aesnet.org
Presentation date :
12/2/2018 4:04:48 PM
Published date :
Nov 5, 2018, 18:00 PM
Authors :
Darshan Mehta, Sunovion Pharmaceuticals Inc.; Andrew Lee, Sunovion Pharmaceuticals Inc.; Jason Simeone, Evidera; Krithika Rajagopalan, Sunovion Pharmaceuticals Inc.; Robert Carroll, Evidera; and Beth Nordstrom, Evidera
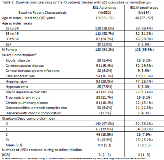
Rationale: Eslicarbazepine acetate (ESL) is a once-daily anti-epileptic drug (AED) approved in the US to treat focal seizure (FS) in patients 4 years and older either as adjunctive or monotherapy. However, there is limited information on the use of ESL in routine clinical practice for adult FS patients. This study aimed to describe the characteristics of adults with FS receiving ESL either as adjunctive or monotherapy. Additionally, health resource utilization (HRU) before and after therapy initiation were compared. Methods: The retrospective cohort study leveraged administrative claims data from the IQVIA PharMetrics Plus database. Adults with evidence of FS and treatment with ESL either as adjunctive or monotherapy between November 2013 and March 2016 were included. Adjunctive therapy patients were defined as those with concurrent treatment with another AED that overlapped with continuous ESL treatment, initiated either on the same day or sequentially (within 30 days). Monotherapy patients were defined as those prescribed ESL without an adjunctive AED regimen during the study period. Patients were included for analysis if they met the following criteria: 12 months of baseline and follow-up data, no evidence of pregnancy during baseline and age >=18 years at start of therapy. Demographic (e.g. age, gender) and clinical characteristics (e.g. comorbidities, prior AEDs received) of patients treated with ESL were studied at baseline. HRU was measured as all-cause or epilepsy-related inpatient (IP), emergency room (ER) or outpatient (OP) hospital/clinic visits. HRU was studied both during baseline and follow up. The change in proportion of patients with >=1 HRU visit from baseline to follow-up was tested for statistical significance using McNemar’s test. Results: From a total of 10,335 adult FS patients, 496 patients received ESL. Of these, 67% (n = 332) were treated with adjunctive therapy, while the rest (n = 164) received monotherapy. In both cohorts median age was 40 years and the majority of patients were female (Table 1). Mean comorbidity index in adjunctive and monotherapy cohort was 0.9 and 1 respectively. Patients treated with adjunctive ESL therapy and monotherapy received a median of 3 and 2 prior AEDs respectively. At baseline, 25.0% and 22.6% of patients in the adjunctive and monotherapy cohort had an epilepsy-related hospitalization, respectively. Compared to baseline, patients in the adjunctive therapy cohort experienced statistically significant reduction for all cause ER (36.1% V. 28.0%, p=0.002) and OP visits (81% V. 75.6%, p=0.036). Patients in the monotherapy cohort experienced statistically significant reduction in all cause HRU categories (p<0.05) and epilepsy related categories: ER (15.9% V. 8.5%; p=0.023) and OP visits (47.6% V. 35.4%, p=0.018) (Table 2). Conclusions: This real world study of adult FS patients treated with ESL, suggests that ESL is used in patients across all adult age groups. Treatment with ESL was associated with reductions in a number of HRU categories for both adjunctive and monotherapy patients. These findings may be an important consideration for patients, physician, and healthcare systems in optimizing treatment of FS. Funding: The study was funded by Sunovion Pharmaceuticals Inc.
.tmb-.png?Culture=en&sfvrsn=9c06d003_0)